Abstract
Background: Evorpacept is a high affinity CD47-blocking fusion protein with an inactive human immunoglobulin Fc region designed to promote phagocytosis of tumor cells in combination with other anti-neoplastic therapies, while adding minimal toxicity. Here, we present results from the phase 1a dose escalation part of the ASPEN-05 study evaluating the safety and tolerability of evorpacept administered in combination with standard venetoclax (VEN) and azacitidine (AZA) in subjects with acute myeloid leukemia (AML).
Methods: ASPEN-05 (NCT04755244) is a phase 1/2 open-label, multicenter study. The phase 1a dose escalation part is designed to evaluate the safety and tolerability and establish the maximum tolerated dose (MTD) of intravenous (IV) evorpacept in combination with VEN and AZA. Adult subjects with relapsed/refractory (R/R) AML or newly diagnosed (ND) AML with adverse risk genetics and considered ineligible for intensive induction therapy were enrolled into cohorts of escalating doses of evorpacept (20 mg/kg Q2W, 30 mg/kg Q2W, and 60 mg/kg Q4W) combined with VEN (400 mg PO daily for up to 28 days) and AZA (75 mg/m2 IV/SC daily for 7 days) in a 28-day treatment cycle. The primary endpoint is the frequency of first cycle dose-limiting toxicities (DLTs). Key secondary endpoints are to characterize the pharmacokinetic (PK) profile of evorpacept in combination with VEN and AZA, and to assess anti-leukemic activity using the ELN 2017 response criteria.
Results: As of July 8, 2022, 14 subjects including 10 males and 4 females with a median age of 71 years (range 50-82) were treated at evorpacept doses of 20 mg/kg Q2W (N=4), 30 mg/kg Q2W (N=4), and 60 mg/kg Q4W (N=6). There were 11 subjects with R/R AML with a median of 1 prior line of therapy (range 1-2), including 9 that were exposed to prior VEN, 2 that were VEN-naïve, and 5 that had received a prior hypomethylating agent. Of the 3 subjects with ND AML, 2 had therapy-related AML, and all had TP53 mutation. Patient characteristics included a median ECOG score of 1 (range 0-2) at the time of screening, and median baseline laboratory values of: creatinine 0.92 mg/L (range 0.54-3.31), total bilirubin 0.7 mg/L (0.3-1.7), platelet count 31.5 x 109/L (range 13-117), and WBC count 2 x 109/L (range 0.4-12.3). Bone marrow studies performed at screening demonstrated a median blast percentage of 27% (range 5-84), 11 subjects with adverse risk and 2 with intermediate risk cytogenetics, and mutations in TP53 (N=10), DNMT3A (N=3), ASXL1, RAS, and RUNX1 (N=2 each).
An MTD of evorpacept was not reached. All subjects experienced an adverse event. Evorpacept-related AEs of nausea, vomiting (n=2 each; 14%), cytokine release syndrome, metabolic acidosis, muscular weakness, and pyrexia (n=1 each; 7%) were observed. Grade ≥3 AEs of any causality occurring in >2 subjects were febrile neutropenia, anemia (n=6 each; 43%), AST increased (n=5; 36%), platelet count decreased (n=4; 29%), and pneumonia (n=3; 21%). There was one reversible Grade 3 evorpacept-related AE of cytokine release syndrome that met criteria for DLT in the 60 mg/kg Q4W cohort. There were no on-study evorpacept-related deaths. Preliminary PK data indicated dose-proportional pharmacokinetics and PD data demonstrated full CD47 target occupancy in both peripheral blood and bone marrow across all dose levels at both evorpacept peak and trough concentrations.
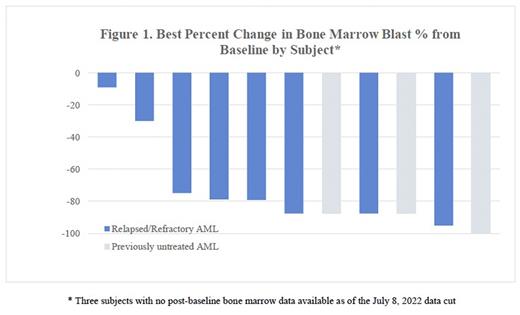
With limited follow up data, in 12 subjects that had a post-baseline disease assessment there was initial evidence of clinical activity observed, with a reduction in bone marrow blasts (Figure 1) across all cohorts and objective responses in 3 ND subjects, 2 R/R VEN-naïve subjects, and 2 R/R VEN-exposed subjects. Results will be updated at the time of presentation.
Conclusions: The addition of evorpacept to standard dose VEN and AZA for AML was well tolerated with no MTD reached. The maximum administered evorpacept dose was 60 mg/kg Q4W, and preliminary dose-proportional pharmacokinetics was seen along with full CD47 target occupancy in both peripheral blood and bone marrow across all dose levels evaluated. Initial anti-leukemic activity was observed in subjects with both ND and R/R AML (including VEN-naïve and prior VEN-exposed disease). These results support further evaluation of evorpacept in myeloid malignancies, including AML.
Disclosures
Garcia-Manero:Novartis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Aprea: Honoraria; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy. Przespolewski:ALX Oncology: Other: Site Principal Investigator; Merck: Other: Site Principal Investigator; Jazz Pharmaceuticals: Research Funding. Abaza:ALX Oncology: Research Funding; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Byrne:Karyopharm: Research Funding; Celularity, Concert: Consultancy, Other: DSMC; CTI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho: Research Funding. Fong:ALX Oncology: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Jin:Elucida Oncology Inc.: Current equity holder in private company; Elicit Therapeutics: Current equity holder in private company. Forgie:ALX Oncology: Current Employment, Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company. Tsiatis:ALX Oncology: Current Employment, Current equity holder in publicly-traded company; Plexxicon Inc.: Ended employment in the past 24 months. Guan:ALX Oncology: Current Employment, Current equity holder in publicly-traded company; Nektar Therapeutics: Current equity holder in publicly-traded company. Erba:Trillium Therapeutics: Consultancy; Takeda: Consultancy; MacroGenics: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Celgene: Consultancy, Other, Speakers Bureau; ImmunoGen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; Janssen Oncology: Consultancy; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Astellas Pharma: Consultancy; Amgen: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Consultancy, Research Funding, Speakers Bureau; Kura Oncology: Consultancy; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.